
Carbon allocation priorities in spruce trees

During climate extremes, trees cannot produce sufficient energy-rich carbon compounds through photosynthesis and they become dependent on stored reserves. Despite the important role of nonstructural carbohydrate (NSC) storage in trees, our current understanding assumes that NSC storage is a low priority sink that accumulates during periods of positive carbon balance, and that can be completely depleted to fuel respiration or growth under negative carbon balance. This widely-held assumption, however, may not be valid if trees prioritize allocation to NSC storage over growth to ensure survival under stress.
To challenge this assumption, we manipulated the carbon balance of Norway spruce (Picea abies) by progressively reducing atmospheric CO2 concentrations ([CO2]) from 400 ppm (control) to 120 ppm and further to 50 ppm, where trees go into negative carbon balance. Using flux measurements, isotopic tracing, targeted metabolomics and transcriptomics, we investigated how limitation of source supply influences carbon allocation to growth and carbon storage, and their molecular regulation in Norway spruce (Picea abies) clones.
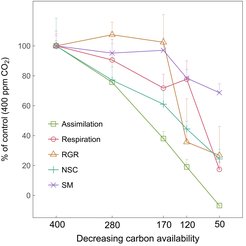
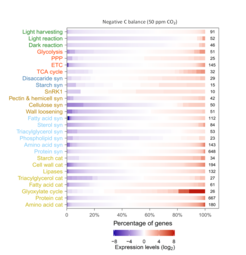
Our results show that under reducing C supply, NSCs were initially mobilized to support respiration, growth and defense. However, after reducing CO2 to 120 ppm (i.e. carbon compensation point) and 50 ppm (i.e. negative carbon balance), trees preferentially maintain operational levels of NSC (ca. 45% and 25% of control) with constant incorporation of newly-assimilated carbon, while reducing growth and then respiration activities. Molecular evidence shows that photosynthesis (e.g. light reactions) and growth (e.g. cellulose synthesis) are downregulated while sucrose and starch biosynthesis pathways are upregulated under negative carbon balance, supporting that trees prioritize carbon allocation to storage over growth. Moreover, trees under negative carbon balance actively increase the turnover rate of starch, lipids and amino acids, most likely to ensure basal respiration (TCA cycle) and mitigate stress.
Our experiment provides mechanistic evidence that trees faced with severe photosynthetic limitation strategically regulate storage allocation and consumption to ensure survival at the expense of growth. Understanding such allocation strategies is crucial for assessing and predicting how trees may respond to extreme events involving steep declines in photosynthesis, like severe drought, or defoliation by heat waves, late frost or insect attack.


