Central Flask Facility
For remote sites and for airborne campaigns, often the only way to obtain precise data is to collect air in glass flasks in situ and to analyze the samples later in a laboratory equipped with the appropriate analytical instruments. Compared to in-situ measurements this increases the risk of altering the air trace gas composition either during the sampling procedure or during the storage prior to measurement.
The composition of an air sample in glass flasks can be adulterated by a number of effects:
- Insufficient flushing before the actual sampling can lead to a contamination by the previous flask content.
- Adsorption and desorption of gas molecules on surfaces can change the concentrations and / or isotopic compositions of O2 , CO2 and other trace gas species, becoming important at high precision levels.
- Polymers used as sealing material are subject to gas permeation thereby reducing the reliability of the seal for long term storage.
- Organic material in the flasks including O-ring material and / or O-ring can partially be oxidized, altering the air composition as well as the isotopic signature in both isotopes of CO2 .
- δ18O values can change when traces of moisture are present in the flask or develop over time (e.g. via permeation through the O-rings).
In order to stabilize the conditions for all flasks that leave our institute for being filled anew with sample air in the field we created a Central Flask Facility in 2001 that handles flasks and keeps track of their whereabouts and performance.
Flask design

To reduce adsorption and desorption of gas molecules on surfaces, the flasks used for air analysis are made of borosilicate glass 3.3 (Schott Duran®). The air sampling is done with flasks of 1L volume equipped with two valves allowing flushing prior the actual sampling. In general the valves are sealed with caps made from Kel-F® (PCTFE, PolyChloroTriFluoroEthylene; Fig. 1). This material shows the lowest permeation of gases (Sturm et al. 2004) compared to most other sealing materials.
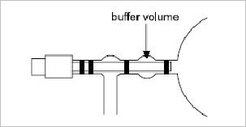
For the less critical case of air emanating from soils, also flasks with PFA (Perfluoralkoxy-Copolymer) O-rings or Viton® (DuPont fluorelastomer) double O-rings with buffer volume (Fig. 2) are in use. These samples usually exhibit a wide range of trace gas mixing ratios and δ13C CO2 values whereas O2 concentration and δ18O values so far are of minor interest. This diminishes relative errors arising from adverse effects of gas permeation and / or oxidation processes of these sealing materials to an acceptable level.
Flask treatment
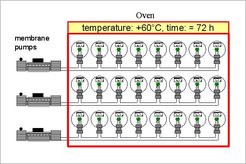
In order to guarantee that the inner surfaces of the flasks are free of contaminations and traces of water originating from the previous sample or from the manufacturing process the flasks routinely are conditioned by keeping them at + 60 °C while evacuating with a low pressure membrane pump. Storage effects on air composition (e.g. δ18O effects) become negligible when this evacuation procedure lasts or exceeds 72 hours. Fig. 3 shows a schematic sketch of the used heating oven. Every flask coming newly into use or suspected to be contaminated is subjected to the described evacuation procedure.
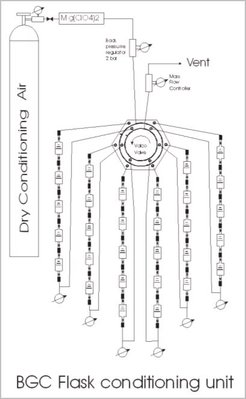
Adsorption and desorption effects can decrease significantly when the differences in air composition and pressure vary only slightly from sample to sample. For this reason the stored flasks are filled with atmospheric air to comply with the conditions during the sampling conditions.
For this purpose we have built a flask conditioning unit (Fig. 4) where all flasks are filled with dry air to a pressure of 1.6 bars by flushing them over a period of 30 min using a flow of 3 l/min (STP). With 5 flasks in a row, these 90 liters of air passing through the flasks replace the air in the flasks about 9 times.
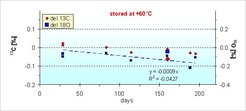
In a long term experiment we could show that the storage temperature has an influence especially on the δ18O of CO2 in flask samples. The δ18O values show a continuous decrease over time at a storage temperature of + 60 °C, whereas this drift was negligible (less than 1/10th) at temperatures at + 25 °C and below in the same time period. Among others we conclude from this observation, that storage at + 60 °C may serve as a quick test to check flask storage performance. Fig. 5 shows the storage pattern of glass flasks after the described treatment of conditioning stored at + 60 °C for extended periods of time. The observed drift in δ18O corresponds to less than 0.01 ‰ in 200 days stored at room temperature. δ13C values are unaffected. This is quite acceptable for the high accuracy required for atmospheric analyses.




