
Messung stabiler Isotope des CO2 in Luft

One approach to get information about the sources and sinks of carbon dioxide (CO2) in the air is to interpret their isotopic ratios of carbon (C) and oxygen (O). Atoms consist of a positively charged nucleus surrounded by negatively charged electrons. More precisely, an atomic nucleus of an element contains a defined number of positively charged protons and a variable number of chargeless neutrons. These isotopes of an element differ in their mass, but the chemical properties are not changed by this. Various physical processes run faster for molecules with lighter isotopes, such as photosynthesis. Therefore, CO2 from different sources (e.g., coal, gas, cement production) contains different characteristic proportions of the various isotopes. The ratios of stable isotopes δ(13C/12C) and δ(18O/16O) in atmospheric CO2 measured in our laboratory may provide an indication to processes that contributed to the concentration of CO2. In addition, an age determination of the carbon in the CO2 can be performed at the Central Radiocarbon Laboratory in Heidelberg using the δ(14C/12C) ratio. Since radioactive 14C in fossil materials has already completely decayed, the fraction of anthropogenically emitted CO2 can thus be identified.
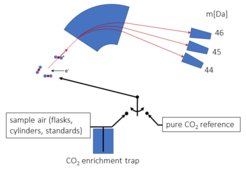
The air samples collected at the measuring stations are analyzed with a special mass spectrometer for the determination of isotope ratios (ThermoFischer MAT253). We have completely automated the measuring instrument and the sample preparation apparatus developed in-house in order to cope with the high sample throughput while maintaining high precision and extensive documentation of the measurement process.
To prepare the measurement, sample air is passed through a cold trap in which the CO2 in the air is frozen out. After thawing, the pure CO2 from the air is fed into the mass spectrometer and ionized with a filament. The positively charged CO2 molecules are focused into a beam via ion-optical elements, passed through a magnet and detected with mass-specific detectors. The mass separation is done with a strong electromagnet which separates the ions according to their mass to charge ratio. From the intensity ratios of the CO2 molecules with masses 44, 45 and 46, the isotope ratios of 13C/12C and 18O/16O can be calculated. All samples are always measured alternately with an internal reference of pure CO2 to achieve the required accuracy. At the same time and under the same conditions, air standards with precisely defined 13C/12C and 18O/16O ratios are also measured with the samples. These air standards define the measurement scale for the measurements.
Isotopic ratios are reported as deviation from a reference standard in the unitless δ-Notation in ‰ =0.001 (δ(13C)=δ(13C/12C) =((13C/12C)sample – (13C/12C)reference)/(13C/12C) reference)) and δ(18O)=δ(18O/16O) =((18O/16O)sample – (18O/16O) reference)/(18O/16O)reference). All measurements are referred to the isotope ratio of CO2 of the reference material, a particular limestone (Vienna Pee Dee Belemnite) dissolved in acid. Pure reference CO2 for δ(13C) and δ(18O) measurements is produced in the Central Calibration Laboratory in a standardized process from the reference material and transferred to a gas mixture equal to the air composition. The WMO Central Calibration Laboratory for δ(3C) and δ(18O) measurements of CO2 in air is the BGC-IsoLab at the MPI for Biogeochemistry in Jena. The agreement of the scales between the measurement networks is regularly checked by interlaboratory comparisons. The same air samples are measured at different laboratories worldwide. The deviation of measurement results should not exceed certain values, which are defined by the WMO Expert Group as compatibility goals. For CO2 in air, the goal is to achieve measurements with a compatibility of δ(13C) to ±0.02‰ = 0.00002 and δ(18O) to ±0.05‰ = 0.00005.
The measurement results are delivered within ICOS to the Atmospheric Thematic Center in Paris/France and made available to the public via the ICOS Carbon Portal in Lund/Sweden.

